花生四烯乙醇胺
| 本條目需要編修,以確保文法、用詞、语气、格式、標點等使用恰当。 (2015年4月23日) |
| 花生四烯乙醇胺 | |
|---|---|
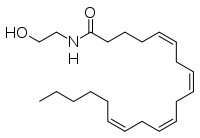 | |
IUPAC名 (5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-tetraenamide | |
| 别名 | N-arachidonoylethanolamine arachidonoylethanolamide |
| 识别 | |
CAS号 | 94421-68-8 |
PubChem | 5281969 |
ChemSpider | 4445241 |
SMILES |
|
InChI |
|
InChIKey | LGEQQWMQCRIYKG-DOFZRALJBA |
ChEBI | 2700 |
MeSH | Anandamide |
IUPHAR配体 | 2364 |
| 性质 | |
化学式 | C22H37NO2 |
摩尔质量 | 347.53 g/mol g·mol⁻¹ |
| 若非注明,所有数据均出自一般条件(25 ℃,100 kPa)下。 | |
花生四烯乙醇胺,亦稱N-花生四烯乙醇胺或AEA ,是一種內源性的大麻醇類神經傳導物質。此命名取自梵文(和印度半島宗教用語﹞的阿難陀(ananda),意为”喜悅、極樂、欣喜”,以及醯胺[1][2]。它是由N-花生四烯酰磷脂酰乙醇胺透過多種途徑合成[3]。花生四烯乙醇胺,主要是由脂肪酸醯胺水解酶(FAAH)將其降解成乙醇胺和花生四烯酸。如此,脂肪酸醯胺水解酶(FAAH)的抑制物導致花生四烯乙醇胺層級的提升,並被用于治療[4][5]。
目录
1 歷史
2 生理功能
3 合成與降解
4 药用价值
5 参见
6 參考文獻
7 外部連結
歷史
其結構最初是由W. A. Devane, Lumír Hanuš等人在1992年時描述。當時他們在耶路撒冷的希伯來大學一個由Raphael Mechoulam帶領的團隊工作。[6]
生理功能
花生四烯乙醇胺可以作用在身體的中樞,例如:在腦部或其周邊;也可以作用在身體的其他部位。這些不同的作用主要是由CB1大麻素受體在中樞神經系統中調節,以及在周圍神經的CB2大麻素受體調控。[7]後者主要是參與免疫系統的功能作用。大麻素受體最初被發現時,被視為對Δ9-四氫大麻酚(Δ9-THC,通常稱為THC)是敏感的,而其中THC是大麻中的主要精神活性物質。大麻素的發現源自於對CB1和CB2的研究,因為這是一個無可避免的內源性化學物質,並且會影響這些受體。花生四烯乙醇胺已經被證實會影響大鼠的工作記憶。[8]而現在的研究正在探討大麻素作用對人類行為的影響,例如:在進食和睡眠的模式,以及疼痛減輕。花生四烯乙醇胺也對早期胚胎中,胚囊著床在子宮時有重要影響。因此大麻素,例如:Δ9-THC,可能在人類妊娠的早期階段影響其過程。[9]花生四烯乙醇胺在血漿中達到高峰時,發生在排卵期,並且和雌二醇及促性腺激素呈現正相關,這表明了以上這些物質可能參與了AEA 的調節。[10]而後,花生四烯乙醇胺曾被提議用來做為不孕症的生物標誌物,但迄今為止在臨床上尚無任何預測值。[11]
花生四烯乙醇胺在攝食行為中扮演著著調節的角色,而神經作用的行為以及產生愉悅的感受也受其影響。此外,將花生四烯乙醇胺直接注入大鼠的前腦正向強化刺激相關區域伏隔核時,會增強大鼠對於所攝取蔗糖的愉悅感,並且會增進食物的攝入量。
在1998年發表的一篇研究中也曾經提到,花生四烯乙醇胺會抑制人類乳腺癌細胞的增殖。[12]
有一些研究表示,花生四烯乙醇胺的釋放為運動誘導之鎮痛作用的機制,特別是在跑步時。[13]
在1996年,研究人員發現巧克力中含有花生四烯乙醇胺。他們也發現,有兩種物質(N-oleoylethanolamine 和 N-linoleoylethanolamine)會模仿花生四烯乙醇胺帶來的影響。[14]
合成與降解
人體可以由N-花生四烯酰磷脂酰乙醇胺(NAPE)合成花生四烯乙醇胺,而N-花生四烯酰磷脂酰乙醇胺(NAPE)本身是透過N-酰基轉移酶,將花生四烯酸從卵磷脂轉移至腦磷脂上的游離胺上。[15][16] 花生四烯乙醇胺由NAPE的合成可以透過多種途徑達成,包括酵素的參與,例如磷脂酶A2,磷脂酶C和NAPE-PLD[3]。
內源性花生四烯乙醇胺並不常存在且其半生期非常短,因為脂肪酸酰胺水解酶(FAAH)的作用,會將其分解成游離的花生四烯酸和乙醇胺。與仔豬相關的研究表明,花生四烯酸及其它必需脂肪酸的攝入量,會影響其腦部花生四烯乙醇胺和其他內源性大麻素的量[17]。而被以高脂肪飲食飼養的小鼠,其肝臟中的花生四烯乙醇胺的量會增加,同時也會增進脂肪的生成[18]。這說明了至少在囓齒動物中,花生四烯乙醇胺對於肥胖的發展會產生影響。
對乙酰氨基酚(acetaminophen)在代謝上,會透過FAAH,去和花生四烯酸結合而形成AM404[19]。此對乙酰氨基酚(acetaminophen)的代謝產物,對於TRPV1香草素受體,是一種強促效劑,但對於CB1和CB2受體,為弱促效劑,而對於花生四烯乙醇胺而言,為再吸收抑制劑。因此,身體和大腦中花生四烯乙醇胺的量提升。以此方式,對乙酰氨基酚(acetaminophen)可作為仿大麻素代謝產物的前體藥物。這個作用可以使得對乙酰氨基酚(acetaminophen)的鎮痛作用受到部分或全部的影響[20][21]
花生四烯乙醇胺和其姊妹分子2-花生四烯酸甘油酯的運輸蛋白已經被鑑定確認了,包括熱休克蛋白(Hsp70s)和脂肪酸結合蛋白(FABPs)。[22][23]
药用价值
英國皇家化學學會曾表示,有研究顯示AM1172有可能被開發成藥物,可以提升大腦的花生四烯乙醇胺,用來治療焦慮以及憂鬱。[24]
参见
- 大麻素
- Virodhamine
四氫大麻酚(THC)- 2-花生四烯酸甘油酯
- 脂肪酸酰胺水解酶
- 運輸大麻
- Raphael Mechoulam
參考文獻
^ Devane, W.; Hanus, L; Breuer, A; Pertwee, R.; Stevenson, L.; Griffin, G; Gibson, D; Mandelbaum, A; Etinger, A; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 18 December 1992, 258 (5090): 1946–1949. doi:10.1126/science.1470919.
^ Mechoulam R, Fride E. The unpaved road to the endogenous brain cannabinoid ligands, the anandamides. (编) Pertwee RG. Cannabinoid receptors. Boston: Academic Press. 1995: 233–258. ISBN 0-12-551460-3.
^ 3.03.1 Wang, Jun; Ueda, Natsuo. Biology of endocannabinoid synthesis system. Prostaglandins & Other Lipid Mediators (Elsevier BV). 2009, 89 (3-4): 112–119. ISSN 1098-8823. doi:10.1016/j.prostaglandins.2008.12.002.
^ Gaetani, Silvana; Dipasquale, Pasqua; Romano, Adele; Righetti, Laura; Cassano, Tommaso; Piomelli, Daniele; Cuomo, Vincenzo. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs.. International review of neurobiology. 2009, 85: 57–72. PMID 19607961. doi:10.1016/S0074-7742(09)85005-8.
^ Hwang, Jeannie; Adamson, Crista; Butler, David; Janero, David R.; Makriyannis, Alexandros; Bahr, Ben A. Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: A neuroprotective therapeutic modality. Life Sciences. April 2010, 86 (15-16): 615–623. doi:10.1016/j.lfs.2009.06.003.
^ Mechoulam, WA; Hanus L; Breuer A; Pertwee RG; Stevenson LA; Griffin G; Gibson D; Mandelbaum A; Etinger A; Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. December 1992, 258 (5090): 1946–9. Bibcode:1992Sci...258.1946D. PMID 1470919. doi:10.1126/science.1470919.
^ Pacher, P. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacological Reviews (American Society for Pharmacology & Experimental Therapeutics (ASPET)). 2006-09-01, 58 (3): 389–462. ISSN 0031-6997. doi:10.1124/pr.58.3.2.
^ allet PE, Beninger RJ; Beninger. The endogenous cannabinoid receptor agonist anandamide impairs memory in rats. Behavioural Pharmacology. 1996, 7 (3): 276–284. doi:10.1097/00008877-199605000-00008.
^ Piomelli D. THC: moderation during implantation. Nat. Med. January 2004, 10 (1): 19–20. PMID 14702623. doi:10.1038/nm0104-19.
^ El-Talatini MR, Taylor AH, Konje JC; Taylor; Konje. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. April 2010, 93 (6): 1989–96. PMID 19200965. doi:10.1016/j.fertnstert.2008.12.033.
^ Rapino, C.; Battista, N.; Bari, M.; Maccarrone, M. Endocannabinoids as biomarkers of human reproduction. Human Reproduction Update. 2014, 20 (4): 501–516. ISSN 1355-4786. PMID 24516083. doi:10.1093/humupd/dmu004.
^ De Petrocellis, Luciano; Melck, Dominique; Palmisano, Antonella; Bisogno, Tiziana; Laezza, Chiara; Bifulco, Maurizio; Di Marzo, Vincenzo. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proceedings of the National Academy of Sciences. 7 July 1998, 95 (14): 8375–8380. PMID 9653194. doi:10.1073/pnas.95.14.8375.
^ 存档副本 (PDF). [2013-12-15]. (原始内容 (PDF)存档于2013-12-15).
^ di Tomaso E, Beltramo M, Piomelli D.; Beltramo; Piomelli. Brain cannabinoids in chocolate. Nature. Aug 1996, 382 (6593): 677–8. PMID 8751435. doi:10.1038/382677a0.
^ Natarajan V, Reddy PV, Schmid PC, Schmid HH; Reddy; Schmid; Schmid. N-Acylation of ethanolamine phospholipids in canine myocardium. Biochim. Biophys. Acta. August 1982, 712 (2): 342–55. PMID 7126608. doi:10.1016/0005-2760(82)90352-6.
^ Cadas H, di Tomaso E, Piomelli D; Di Tomaso; Piomelli. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J. Neurosci. February 1997, 17 (4): 1226–42. PMID 9006968.
^ Berger, Alvin; Crozier, Gayle; Bisogno, Tiziana; Cavaliere, Paolo; Innis, Sheila; Di Marzo, Vincenzo. Anandamide and diet: Inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proceedings of the National Academy of Sciences. 15 May 2001, 98 (11): 6402–6406. PMID 11353819. doi:10.1073/pnas.101119098.
^ Osei-Hyiaman, Douglas; DePetrillo, Michael; Pacher, Pál; Liu, Jie; Radaeva, Svetlana; Bátkai, Sándor; Harvey-White, Judith; Mackie, Ken; Offertáler, László; Wang, Lei; Kunos, George. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. Journal of Clinical Investigation. 2 May 2005, 115 (5): 1298–1305. PMID 15864349. doi:10.1172/JCI23057.
^ Högestätt, Edward D.; Jönsson, Bo A. G.; Ermund, Anna; Andersson, David A.; Björk, Henrik; Alexander, Jessica P.; Cravatt, Benjamin F.; Basbaum, Allan I.; Zygmunt, Peter M. Conversion of Acetaminophen to the BioactiveN-Acylphenolamine AM404 via Fatty Acid Amide Hydrolase-dependent Arachidonic Acid Conjugation in the Nervous System. Journal of Biological Chemistry (American Society for Biochemistry & Molecular Biology (ASBMB)). 2005-06-29, 280 (36): 31405–31412. ISSN 0021-9258. doi:10.1074/jbc.m501489200.
^ Bertolini, Alfio; Ferrari, Anna; Ottani, Alessandra; Guerzoni, Simona; Tacchi, Raffaella; Leone, Sheila. Paracetamol: New Vistas of an Old Drug. CNS Drug Reviews. September 2006, 12 (3-4): 250–275. PMID 17227290. doi:10.1111/j.1527-3458.2006.00250.x.
^ Sinning, Christian; Watzer, Bernhard; Coste, Ovidiu; Nüsing, Rolf M.; Ott, Ingo; Ligresti, Alessia; Marzo, Vincenzo Di; Imming, Peter. New Analgesics Synthetically Derived from the Paracetamol Metabolite-(4-Hydroxyphenyl)-(5,8,11,14)-icosatetra-5,8,11,14-enamide. Journal of Medicinal Chemistry. 25 December 2008, 51 (24): 7800–7805. PMID 19053765. doi:10.1021/jm800807k.
^ Kaczocha, M.; Glaser, S.T.; Deutsch, D.G. Identification of intracellular carriers for the endocannabinoid anandamide. Proceedings of the National Academy of Sciences of the United States of America. 2009, 106 (15): 6375–6380. PMC 2669397. PMID 19307565. doi:10.1073/pnas.0901515106.
^ Oddi, S.; Fezza, F.; Pasquariello, N.; D'Agostino, A.; Catanzaro, G.; De Simone, C.; Rapino, C.; Finazzi-Agro, A.; Maccarrone, M. Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chemistry & Biology. 2009, 16 (6): 624–632. PMID 19481477. doi:10.1016/j.chembiol.2009.05.004.
^ Evans, Jon. Easing anxiety with anandamide. Chemistry World. July 2004.
外部連結
Could anandamide be the missing link to "runner's high"? Accessed 2008-10-18
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||
