COPII
| Sec23 homolog A | |
|---|---|
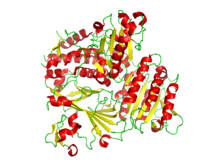 Ribbon diagram of the crystallographic structure of the COPII heterodimer of Sec23 and Sec24. Alpha helices are in red and the beta sheets are in yellow.[1] | |
| Identifiers | |
| Symbol | SEC23A |
| Entrez | 856311 |
| HUGO | 10701 |
| OMIM | 610511 |
| PDB | 1M2V |
| RefSeq | NM_006364 |
| UniProt | Q15436 |
| Other data | |
| Locus | Chr. 14 q21.1 |
| SEC24 family, member A | |
|---|---|
| Identifiers | |
| Symbol | SEC24A |
| Entrez | 10802 |
| HUGO | 10703 |
| OMIM | 607183 |
| PDB | 1M2V |
| RefSeq | XM_001132082 |
| UniProt | O95486 |
| Other data | |
| Locus | Chr. 5 q31.1 |
COPII is a coatomer, a type of vesicle coat protein that transports proteins from the rough endoplasmic reticulum to the Golgi apparatus.[2][3] This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI protein. The name "COPII" refers to the specific coat protein complex that initiates the budding process. The coat consists of large protein subcomplexes that are made of four different protein subunits.
Contents
1 Coat proteins
2 Budding process
3 Conformational changes
4 See also
5 References
Coat proteins
There are two protein heterodimers that form the coat complex. These proteins are
Sec23p/Sec24p Heterodimer
Sec13p/Sec31p Heterotetramer
These proteins alone are not able to cause the budding of the vesicle or direct the vesicle to the correct target membrane. SNARE, cargo, and other proteins are also needed for these processes to occur. The CopII protein does, however, cause the binding that forms vesicle coat, and thereby causes the release from the ER. The exact process of how the vesicle is brought to a particular location, or how that location is determined is not yet known.
Budding process
The GTPase Sar1p is a protein that hydrolyzes GTP and acts like a molecular "switch" that flips between an activated and membrane embedded GTP-bound form, and inactive and soluble GDP-bound form.[4] Inactive GDP-bound Sar1p is attracted to the cytosolic side of the endoplasmic reticulum. Sec12, a transmembrane protein found in the ER acts as a Guanine nucleotide exchange factor by stimulating the release of GDP to allow the binding of GTP. Now in a GTP bound state, Sar1p undergoes a conformational change which exposes a hydrophobic tail that can be inserted into the lipid bilayer, binding it to the membrane. Membrane-bound Sar1p recruits the Sec23p/24p complex to form what is collectively known as the pre-budding complex. The pre-budding complex recruits the long, flexible Sec13p/31p complex. Sec13p/31p complexes polymerizes on the cytosolic side of the membrane to form a convex mesh structure. The assembling mesh causes the membrane to bulge outward until a vesicle buds off. Some proteins are found to be responsible for selectively packaging cargos into COPII vesicles. For example, Erv29p in Saccharomyces cerevisiae is found to be necessary for packaging glycosylated pro-α-factor.[5]
Conformational changes
CopII has three specific binding sites that can each be complexed. The adjacent picture (Sed5) uses the Sec22 t-SNARE complex to bind. This site is more strongly bound, and therefore is more favored. (Embo)
.mw-parser-output .mod-gallery{display:table}.mw-parser-output .mod-gallery-default{background:transparent;margin-top:0.5em}.mw-parser-output .mod-gallery-center{margin-left:auto;margin-right:auto}.mw-parser-output .mod-gallery-left{float:left}.mw-parser-output .mod-gallery-right{float:right}.mw-parser-output .mod-gallery-none{float:none}.mw-parser-output .mod-gallery-collapsible{width:100%}.mw-parser-output .mod-gallery .title{display:table-row}.mw-parser-output .mod-gallery .title>div{display:table-cell;text-align:center;font-weight:bold}.mw-parser-output .mod-gallery .main{display:table-row}.mw-parser-output .mod-gallery .main>div{display:table-cell}.mw-parser-output .mod-gallery .caption{display:table-row;vertical-align:top}.mw-parser-output .mod-gallery .caption>div{display:table-cell;display:block;font-size:94%;padding:0}.mw-parser-output .mod-gallery .footer{display:table-row}.mw-parser-output .mod-gallery .footer>div{display:table-cell;text-align:right;font-size:80%;line-height:1em}.mw-parser-output .mod-gallery .gallerybox .thumb img{background:none}.mw-parser-output .mod-gallery .bordered-images img{border:solid #eee 1px}.mw-parser-output .mod-gallery .whitebg img{background:#fff!important}.mw-parser-output .mod-gallery .gallerybox div{background:#fff!important}

Conformation of the CopII protein complexed with the snare protein Bet1 (PDB: 1PCX).[6]

Conformation of the CopII protein that is complexed with the snare protein Sed5 (PDB: 1PD0).[6]
See also
COPI vesicles
Clathrin vesicles
References
^ PDB: 3EH1; Mancias JD, Goldberg J (November 2008). "Structural basis of cargo membrane protein discrimination by the human COPII coat machinery". EMBO J. 27 (21): 2918–28. doi:10.1038/emboj.2008.208. PMC 2580787. PMID 18843296..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Lee MC, Miller EA (August 2007). "Molecular mechanisms of COPII vesicle formation". Semin. Cell Dev. Biol. 18 (4): 424–34. doi:10.1016/j.semcdb.2007.06.007. PMID 17686639.
^ Hughes H, Stephens DJ (February 2008). "Assembly, organization, and function of the COPII coat". Histochem. Cell Biol. 129 (2): 129–51. doi:10.1007/s00418-007-0363-x. PMC 2228377. PMID 18060556.
^ Bonifacino, Juan S.; Glick, Benjamin S. (2004-01-23). "The mechanisms of vesicle budding and fusion". Cell. 116 (2): 153–166. doi:10.1016/s0092-8674(03)01079-1. ISSN 0092-8674. PMID 14744428.
^ Belden WJ and Barlowe C. Role of Erv29p in Collecting Soluble Secretory Proteins into ER-Derived Transport Vesicles. Science. 2011.
^ ab 1PCX; 1PD0; Mossessova E, Bickford LC, Goldberg J (August 2003). "SNARE selectivity of the COPII coat". Cell. 114 (4): 483–95. doi:10.1016/S0092-8674(03)00608-1. PMID 12941276.


