Propionate
 | |
| Names | |
|---|---|
IUPAC name Propionate | |
| Other names Propanoate, Propanoic acid, ion(1-) | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChemSpider |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C3H5O2− |
| Appearance | Colorless, oily liquid[dubious ] |
Density | 0.993 g/mL at 20°C[1][dubious ] |
Melting point | −21.5 °C (−6.7 °F; 251.7 K)[1][dubious ] |
Boiling point | 141.1 °C (286.0 °F; 414.2 K)[1][dubious ] |
| Hazards | |
| Main hazards | Flammable, Corrosive |
Flash point | 52 °C (126 °F; 325 K)[1][dubious ] |
Autoignition temperature | 465 °C (869 °F; 738 K)[1][dubious ] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
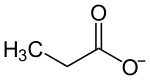
The propionate, or propanoate ion, is C2H5COO− (the conjugate base of propionic acid).
A propionic, or propanoic, compound is a small salt or ester of propionic acid. In these compounds, propionate is often written in shorthand, as CH3CH2CO2 or simply EtCO2.
Propionates should not be confused with propenoates (commonly known as acrylates), the ions/salts/esters of propenoic acid (also known as 2-propenoic acid or acrylic acid).
Propionate is observed to be among the most common short-chain fatty acids produced by human gut microbiota in response to indigestible carbohydrates (fiber) in the diet.[2] A study in mice suggests that propionate is produced by the bacteria of the genus Bacteroides in the gut, and that it offers some protection against Salmonella there.[3] Another study finds that fatty acid propionate can calm the immune cells that drive up blood pressure, thereby protecting the body from damaging effects of high blood pressure.[4]
Examples
Sodium propionate, NaC2H5CO2
Methyl propionate, (C2H5(CO)OCH3)
Calcium propionate, Ca(C2H5CO2)2
Potassium propionate, KC2H5CO2
Fluticasone propionate, C25H31F3O5S
References
^ abcde "propionate | C3H5O2- - PubChem". PubChem..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ How gut microbes talk to organs: The role of endocrine and nervous routes. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5004142 | Section 2.1, paragraph 2: The microbial fermentation of carbohydrates in the gut typically produces acetate, propionate, butyrate, and lactate, which are specific SCFAs.
^ http://med.stanford.edu/news/all-news/2018/07/gut-bacteria-byproduct-protects-against-salmonella.html
^ Ballaschk, Martin. "How dietary fiber and gut bacteria protect the cardiovascular system". Max Delbrück Center for Molecular Medicine.The fatty acid propionate helps defend against the effects of high blood pressure, ... Gut bacteria produce the substance – which calms the immune cells that drive up blood pressure – from natural dietary fiber
This article about an organic compound is a stub. You can help Wikipedia by expanding it. |
